ADVANCING CARE TO OPTIMIZE OUTCOMES in medically supervised settings
At Talphera, we are focused on the development and commercialization of innovative therapies for use in medically supervised settings. Grounded in the understanding that patient outcomes depend on the quality of the tools available to a patient’s medical team, our product candidates are designed to ensure the best care to patients while offering improved cost and convenience for healthcare providers.
When we founded our company, formerly known as AcelRx Pharmaceuticals, our mission was to deliver innovative acute pain products to healthcare providers in the medically supervised setting. With the divestment of our acute pain product in 2023, we embarked on a broader mission to develop and commercialize innovative products for medically supervised settings. In recognition of this new era, we renamed our company Talphera. The name was derived from “Talisman”, meaning a strong leader, and reflects the new “pharmaceutical era” for the company. Our mission at Talphera is to support healthcare providers by developing and commercializing products in medically supervised settings that deliver advances in care to patients.
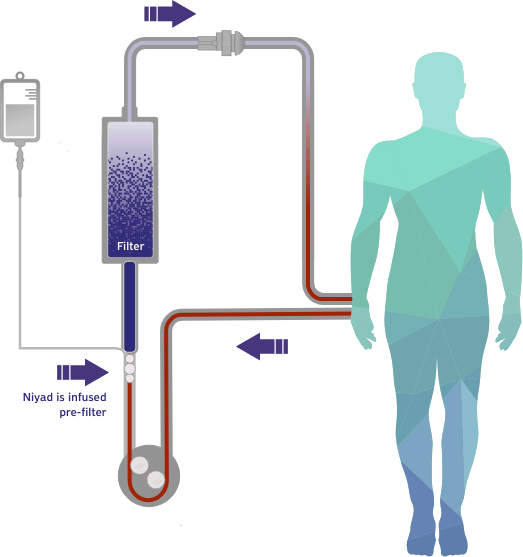
Our lead candidate, Niyad™ (nafamostat mesylate), is positioned to be the first FDA-approved regional anticoagulant for use in the extracorporeal circuit with the potential to meaningfully improve the standard of care for patients undergoing renal replacement therapy (RRT).
OUR PIPELINE
Nafamostat product candidates
Niyad™
Anticoagulation of the extracorporeal circuit – CRRT/IHD (regulated as a device)
Top-line data from clinical study expected by end of 2025
LTX-608™
Various indications – regulated as pharmaceuticals*
Pre-filled syringe product candidates
Fedsyra™
Ephedrine 10 ml ready to use pre-filled syringe of ephedrine hydrochloride
PFS-02
Phenylephrine 10 ml ready to use pre-filled syringe of phenylephrine hydrochloride
*Post-toxicology, expect to be in phase 3 development
OUR PORTFOLIO
Nafamostat: A pipeline-in-a-product
Nafamostat is a broad-spectrum serine protease inhibitor with anticoagulant, anti-inflammatory, and potential antiviral properties with a variety of applications in medically supervised settings.1 Our lead product candidate, Niyad™, a lyophilized form of nafamostat, leverages the ultrashort half-life of nafamostat and its potent anticoagulant effects for potential use as an anticoagulant in dialysis in patients who cannot tolerate heparin or who have an increased risk of bleeding.
In addition to the decades of use in Japan and South Korea as an anticoagulant for dialysis circuits, acute pancreatitis, and disseminated intravascular coagulation, nafamostat has been widely studied for many applications, including as an anticoagulant for the extracorporeal circuit. A meta-analysis published in 2022 compared the safety and efficacy of the use of nafamostat in the extracorporeal circuit against conventional therapies and demonstrated that nafamostat provided significant safety and efficacy improvements over those current standards of care.2

NIYAD™:
Targeting a new standard of care for
use in renal replacement therapy (“RRT”).
Heparin, a systemic anticoagulant with a variable half-life of 1 to 3 hours, has long been the standard anticoagulant for use in dialysis. However, heparin is not safe for patients with a high risk of bleeding or heparin intolerance, leaving limited alternatives for this large patient population.3
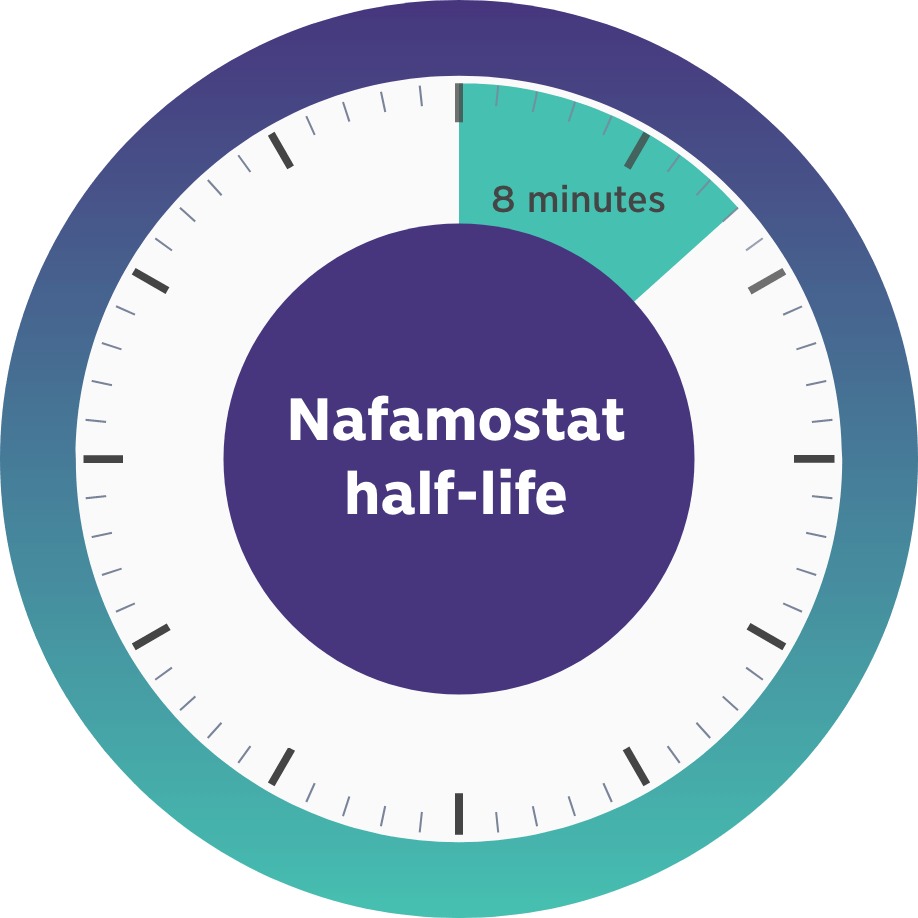
This gap in FDA-approved products led us to develop Niyad, a regional anticoagulant product candidate for injection into the extracorporeal circuit during RRT. In contrast to systemic anticoagulation that anticoagulates both the circuit and patient, regional anticoagulation with nafamostat is expected to operate mainly in the extracorporeal circuit. Given nafamostat’s ultrashort, 8-minute half-life, we believe this anticoagulant agent may be uniquely suited for anticoagulation within the dialysis circuit and has the potential to provide numerous benefits relative to the current standard of care.4
Key benefits of Niyad
- Niyad granted FDA breakthrough device designation status for use as a regional anticoagulant in patients receiving RRT who cannot tolerate heparin or are at a higher risk of bleeding.
- Nafamostat expected to act as a regional anticoagulant in the circuit, enabling FDA regulation of Niyad as a device
- Nafamostat has an 8-minute half-life, resulting in rapid clearance from the blood
- Niyad would be the first FDA-approved regional anticoagulant for the extracorporeal circuit
LTX-608:
Broad therapeutic potential in inflammatory disease
In addition to Niyad, we plan to investigate nafamostat’s broad anti-inflammatory and antiviral potential for the treatment of multiple conditions, including COVID-19, disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS), and acute pancreatitis.
LTX-608 potential applications
DIC is an approved indication for nafamostat in Japan and South Korea
Various ex-US studies have demonstrated positive results for the use of nafamostat as a COVID antiviral agent, and several publications support development of nafamostat as a potential COVID treatment by inhibiting TMPRSS2, a potent broad-spectrum serine protease inhibitor that blocks protease-activation of the viral spike protein 1
A life-threatening lung injury that allows fluid to leak into lungs; nafamostat's potential modes of action of anticoagulation, anti-inflammation and sustaining endothelial barrier function/preventing vascular leak could support exploring development
Approved indication in Japan and South Korea
PREFILLED SYRINGES:
A safer, more economical way to deliver intraoperative medications
Injectable ephedrine and phenylephrine, antihypotensive drugs that healthcare providers rely on every day in perioperative and acute-care settings, have historically only been available in vials or ampules. Talphera, in partnership with the France-based pharmaceutical company Laboratoire Aguettant, is developing 2 prefilled syringe candidates, Fedsyra™ (ephedrine) and PFS-02 (phenylephrine), that are designed to streamline care in clinical settings.
Fedsyra
Fedsyra is a 10-mL ready-to-use prefilled syringe of ephedrine hydrochloride in development for the treatment of hypotension during general anesthesia.
PFS-02
PFS-02 is a 10-mL ready-to-use prefilled syringe of phenylephrine hydrochloride in development for the treatment of hypotension due to vasodilation, as well as for acute pancreatitis and clotting diseases.

DSUVIA
DSUVIA, developed by Talphera and approved by the FDA in November 2018, was divested to Alora Pharmaceuticals in April 2023. Under the terms of the divestment, Alora gives Talphera a 15% royalty on commercial net sales, a 75% royalty on net sales to the United States Department of Defense, and up to $116.5 million in sales-based milestone payments.
To learn more about DSUVIA, please visit DSUVIA.com.
OUR LEADERSHIP
Our management team has decades of experience with successfully developing and commercializing innovative products for healthcare providers and patients.
Management Team
Board of Directors
CAREERS
At Talphera, we understand that our employees are central to our mission to give healthcare providers the best tools to improve patient outcomes. We are looking for employees with exceptional drive, commitment, and a collaborative spirit. If you are a highly skilled, dynamic person who is passionate about improving the lives of patients, we encourage you to explore our career offerings. We offer competitive compensation and benefits, and we recognize outstanding performance.
Talphera is proud to be an equal opportunity employer and prohibits unlawful discrimination based on race, color, religion, gender, sexual orientation, gender identity or expression, national origin or ancestry, age, disability, genetic information, amnesty, or marital or veteran status in accordance with applicable federal, state, and local laws.
Questions about our career opportunities? We’d love to help.
Feel free to reach out to info@talphera.com
CONTACT
For inquiries regarding our products, clinical trials, investment and partnership opportunities, or careers, email us at info@talphera.com.
Address:
1850 Gateway Drive, Suite 175
San Mateo, CA 94404
Phone: 1-650-216-3500
PARTNERSHIPS
Our company is committed to delivering best-in-class drug products and medical devices to healthcare providers in the medically supervised setting. We welcome the opportunity to talk with potential commercialization partners worldwide about licensing relationships, development collaborations, and strategic alliances that complement and extend our own development and commercial capabilities.
To explore potential partnership opportunities, please contact our business development team directly by emailing businessdevelopment@talphera.com.
References
- Niemeyer BF, Miller CM, Ledesma-Feliciano C, et al. Broad antiviral and anti-inflammatory efficacy of nafamostat against SARS-CoV-2 and seasonal coronaviruses in primary human bronchiolar epithelia. Nano Sel. 2022;3(2):437-449. doi:10.1002/nano.202100123
- Lin Y, Shao Y, Liu Y, et al. Efficacy and safety of nafamostat mesilate anticoagulation in blood purification treatment of critically ill patients: a systematic review and meta-analysis. Ren Fail. 2022 Dec;44(1):1263-1279. doi: 10.1080/0886022X.2022.2105233.
- Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin Cardiol. 2019;42(8):774-782. doi:10.1002/clc.23196
- Choi JY, Kang YJ, Jang HM, et al. Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial. Medicine (Baltimore). 2015;94(52):e2392. doi:10.1097/MD.0000000000002392



